how does a car battery work chemistry
Most car batteries rely on a lead-acid chemical reaction to get things moving and grooving. How do car batteries work.

How A Car Battery Works The Engineering Mindset
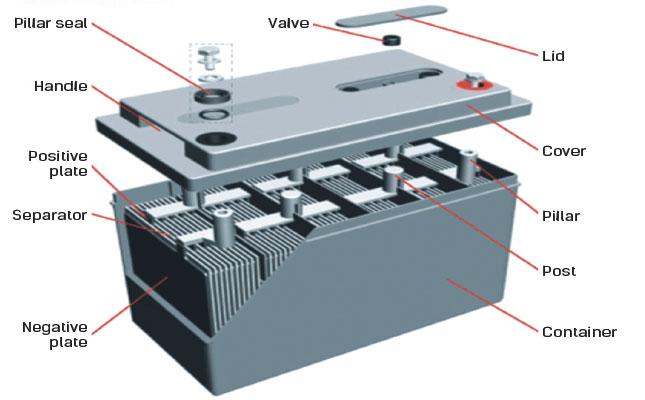
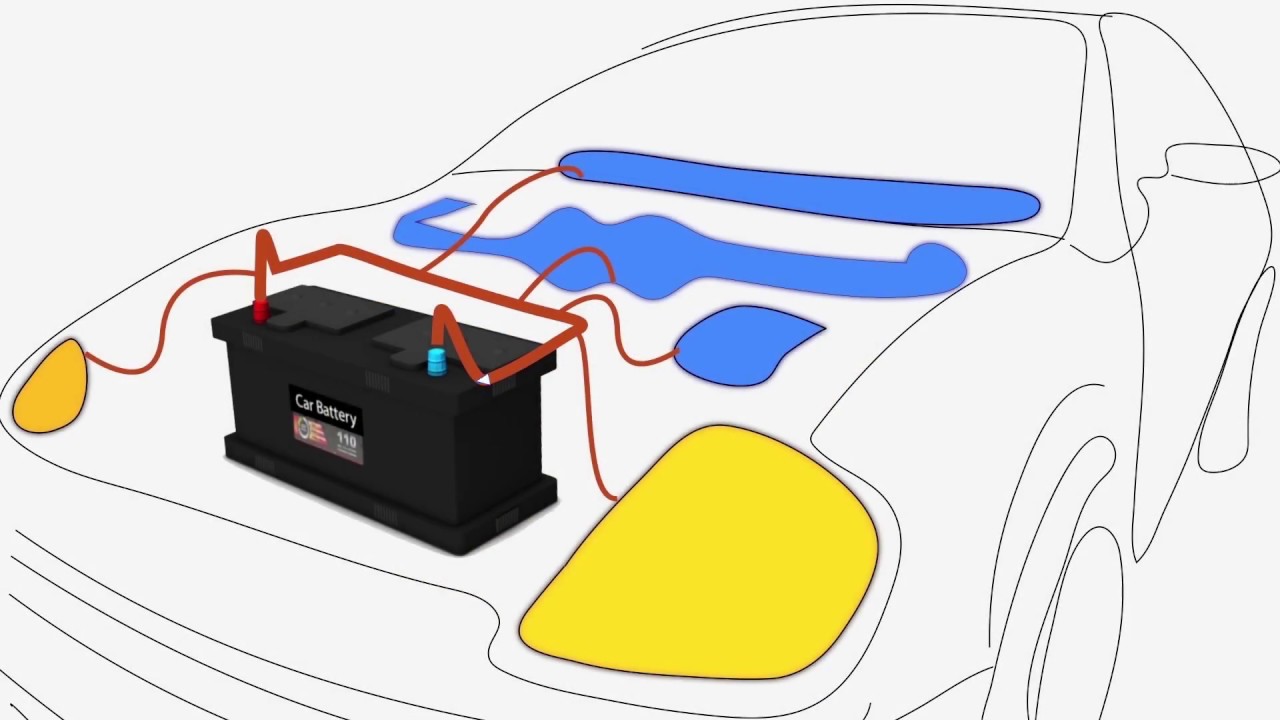
These 6 cells are laid out in a series connection which supplies a total output of 12 volts.
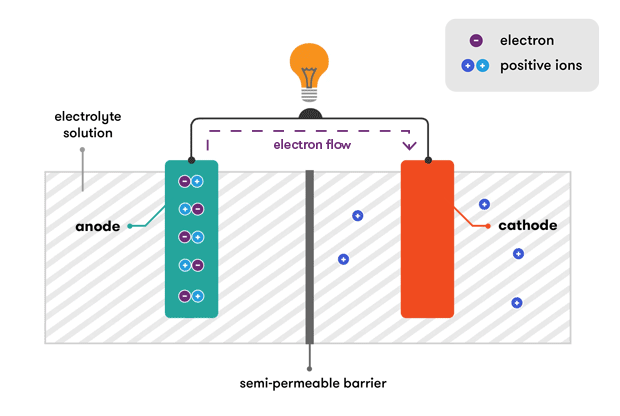
. A battery is made up of an anode cathode separator electrolyte and two current collectors positive and negative. But in case of automobiles the charging source is. There are 2 connectors that go out of the battery.
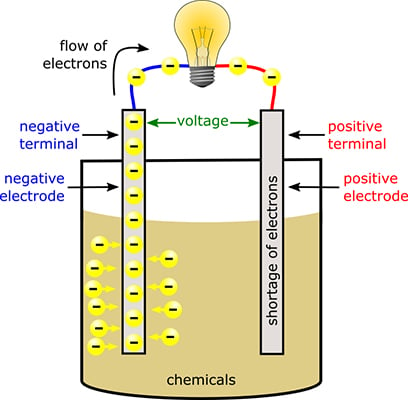
Lead-acid battery rechargeable. The chemical reactions in a battery involve the flow of electrons from one material electrode to another through an external circuit. Inside the battery are 3 important things.
Get the Battery You Need Today. SLI stands for starting lighting and ignition This type of battery provides short bursts of energy in order to power your lights accessories and engine. SLI stands for starting lighting.
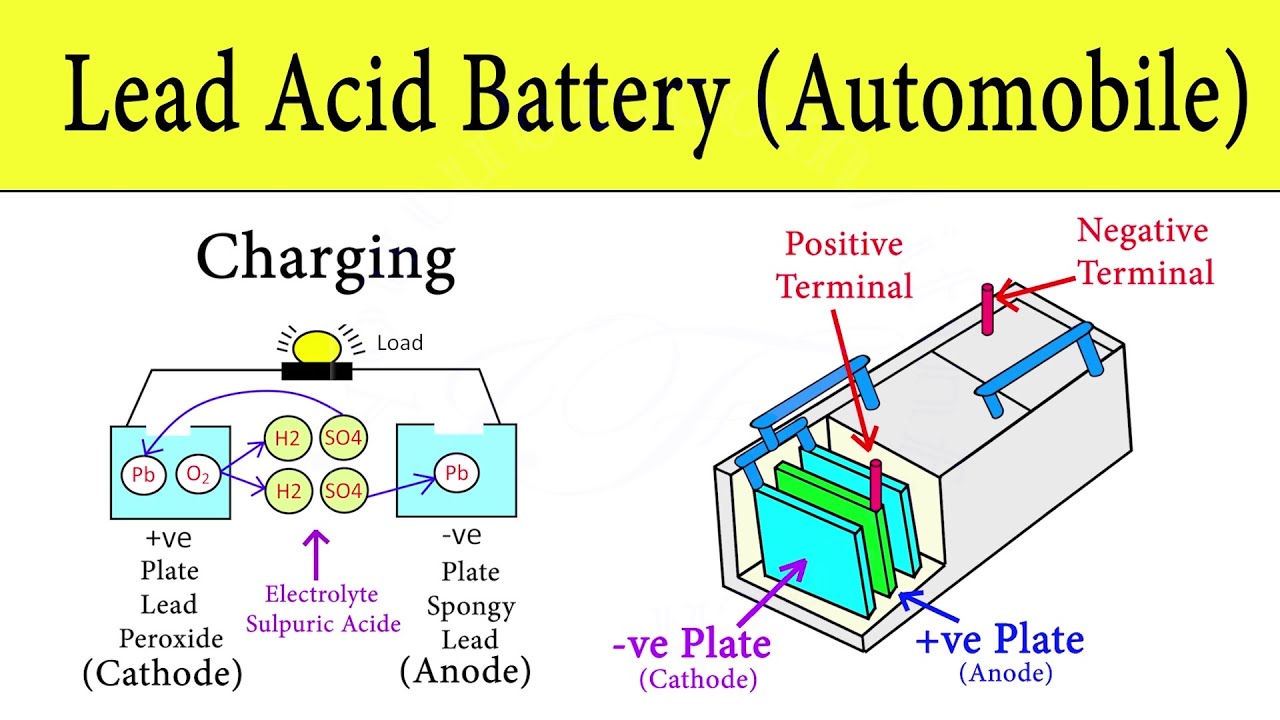
For domestic use this input is ac mains which is converted to dc for charging. To balance the flow of electrons charged ions also flow through an electrolyte solution that is in contact with both electrodes. One is made of lead dioxide and the other of lead.
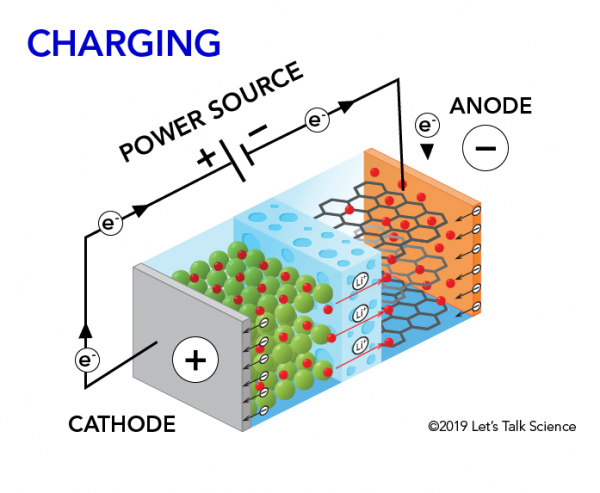
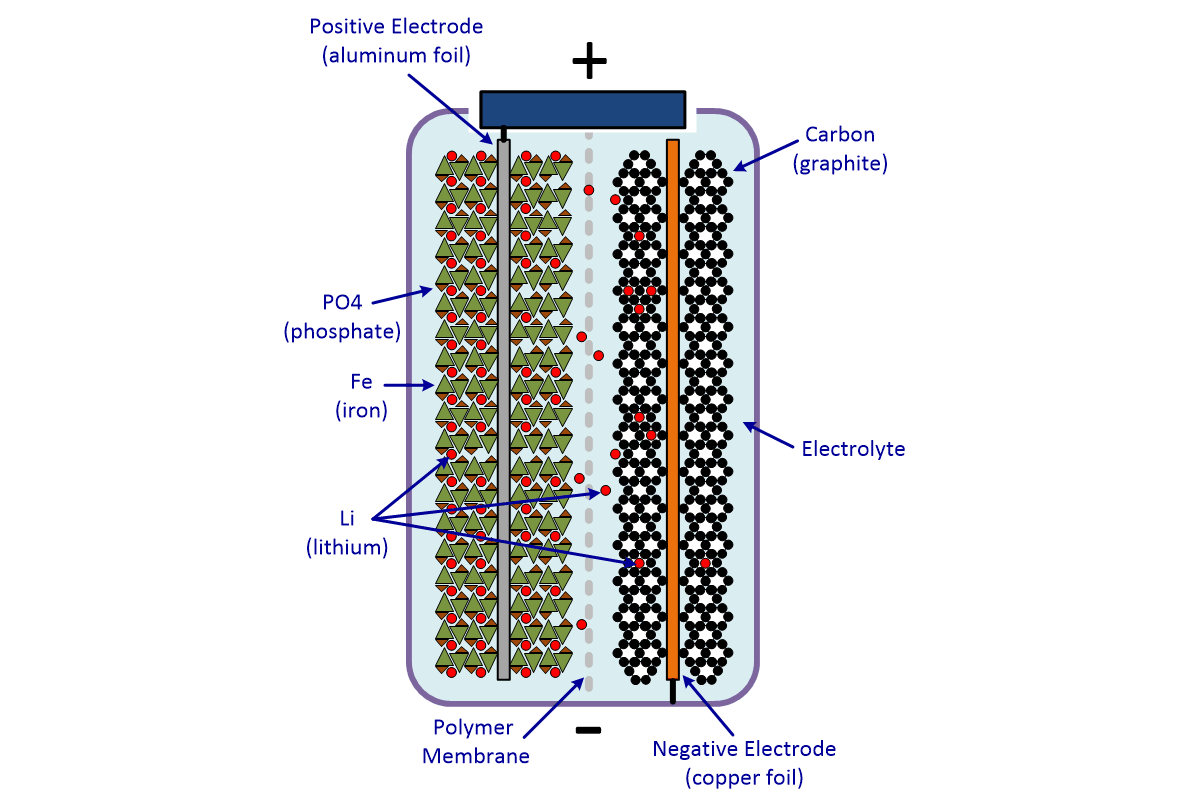
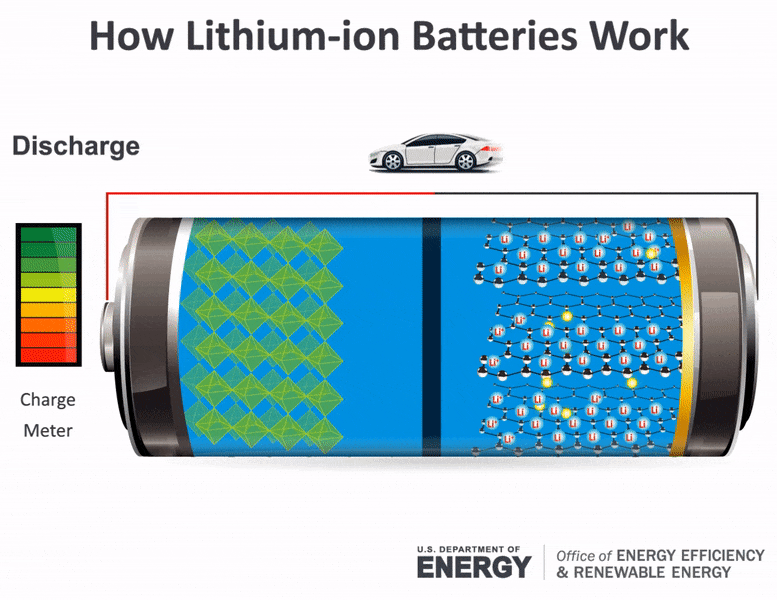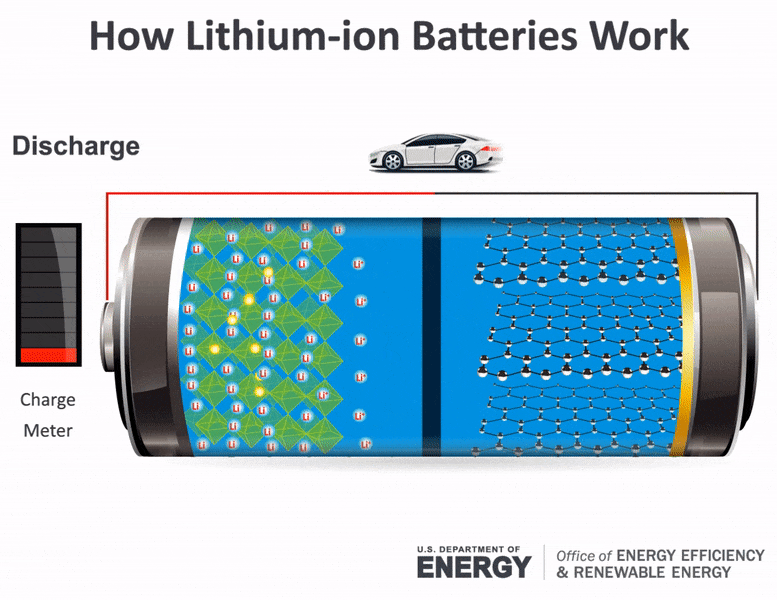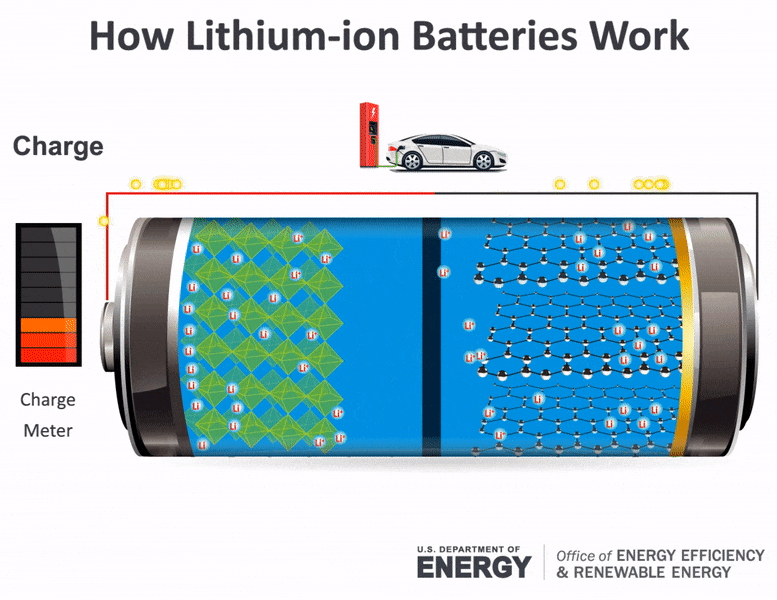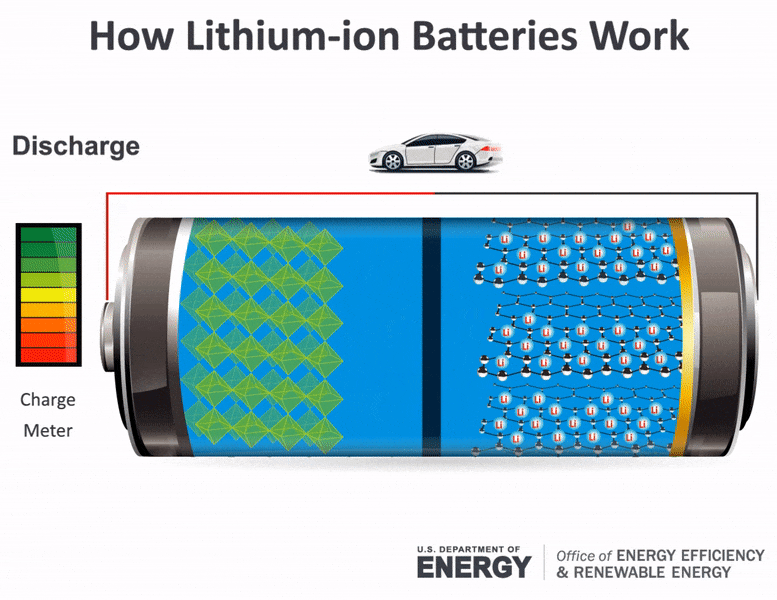
The flow of electrons provides an electric current that can be used to do work. How does a car battery work learn from the basics where we use and battery and how batteries work. The electrolyte carries positively charged lithium ions from the anode to the cathode and vice versa through the separator.
Here is the chemistry involved in car batteries. Most standard car batteries contain six cells that are situated in a row inside the plastic casing. With thanks to Squarespace for sponsoring this video.
Car Battery How it Works video covers explanation what is car battery and how it works. This causes the voltages of each battery to add. The electrodes are usually made of lead dioxide and metallic lead while the electrolyte is a sulfuric acid solution.
Each cell of the battery is made of two plates. The anode and cathode store the lithium. A car battery uses lead-acid technology to turn chemical energy into electricity.
And the electrolyte which separates these terminals. These batteries fall into the SLI category. This is what allows a car battery to be recharged over and over.
Typically the SLI battery is composed of 6 cells each cell delivers 2 volts of electromotive force. Hydrogen H Oxygen O 2 Lead Pb Sulfur S Connection of an external consumer starts the chemical reaction in the battery. Once your car engine starts a current flows through it that re-forms the plates from the lead sulfate and hydrogen.
The general way that a battery works is that when an electronic circuit is connected to the battery electrons are allowed to flow. The movement of the lithium ions creates free electrons in the anode which. A car battery is actually 6 smaller batteries that are lined up in series.
How does a car battery work chemistry. The chemical components of a car battery also known as a lead-acid battery are lead dioxide PbO2 in the cathode lead Pb in the anode and the solution is sulphuric acid H2SO4. All battery work on the same principle of storing dc voltage and supplying to load on demand.
AutoZone Has You Covered at Americas 1 Battery Destination. Once the battery jolts the engine to life. This chemical reaction also works in reverse.
The electrolyte is a chemical medium that allows the flow of electrical charge between the cathode and anode. The best way to understand these reactions is to see them for yourself. When a device is connected to a battery a light bulb or an electric circuit chemical reactions occur on the electrodes that create a flow of electrical energy to the device.
Ad Need a New Battery. The storing is done by input dc voltage. This is the chemistry used in a typical car battery.
Free Curbside Pickup Available When You Shop Online. There are three main components of a battery. It accomplishes this through the usage of cells which contain and store the energy until needed.
How does a car battery work chemistry. Lithium-ion batteries function on the same basic principles but arrive at the chemical reaction in a different way. Two terminals made of different chemicals typically metals the anode and the cathode.
In video you will see chemical reaction between positive and negati. When the battery is in use lead oxide in the cathode reacts with sulphuric acid to form lead sulfate PbSO4.
Electric Car Battery Chemistry What S The Difference Carexpert
Car Battery How It Works Youtube

Battery 101 How Does A Car Battery Work Firestone Complete Auto Care
Lead Acid Battery How Car Battery Works Automobile Battery Working Principle Animation Youtube
Science Made Simple What Are Batteries And How Do They Work

How A Car Battery Works The Engineering Mindset
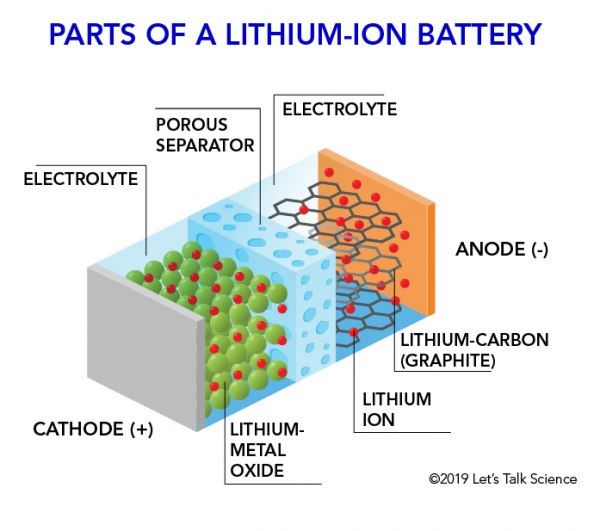
How Does A Lithium Ion Battery Work Let S Talk Science

How Do Batteries Work A Galco Tv Tech Tip Youtube

How A Car Battery Works The Engineering Mindset

How A Car Battery Works The Engineering Mindset
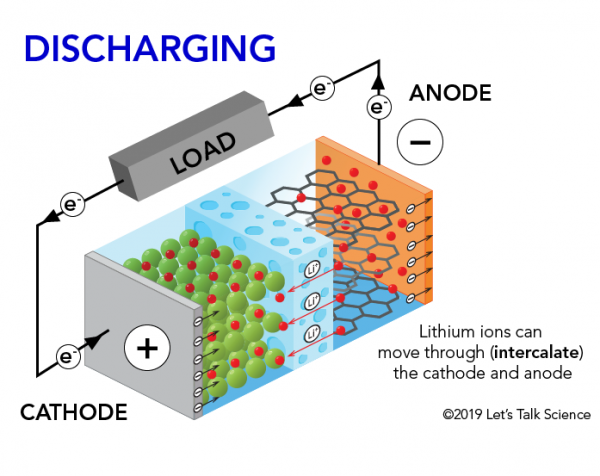
How Does A Lithium Ion Battery Work Let S Talk Science

How Does A Car Battery Work Mach 1 Services

Working Of Car Batteries Askiitians Blog One Place For All Updates On Iit Jee Medical Exams